Answer:
1. a) 1.27 mol H₂SO₄
2. b) 19 g KCl
Step-by-step explanation:
The grass method is an acronym for Given, Required, Application, Solution, Statement.
1. a) Given, 125g H₂SO₄; Required, find the moles H₂SO₄; Application, 125g x molar mass of H₂SO₄, Solution,
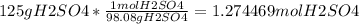 Statement, Round to the lowest number of significant figures to get 1.27 mol H₂SO₄;
Statement, Round to the lowest number of significant figures to get 1.27 mol H₂SO₄;
2. b) I am only show the equation for this one, but it is basically the same as the previous one. The molar mass of KCl is 74.55 g/mol.
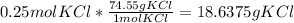
Round to the lowest number of significant figures which is two to get 19 g KCl.
For 3 the unit converter between moles and molecules is 6.022 x 10²³ molecules/mol.
Let me know if you need more help.