Answer:
The acceleration is 3.16x10¹⁷ m/s².
Step-by-step explanation:
First, we need to find the magnitude of the Coulombs force (F):
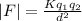
Where:
K is the Coulomb constant = 9x10⁹ Nm²/C²
q₁ is the charge = 20x10⁻⁹ C
q₂ is the electron's charge = -1.6x10⁻¹⁹ C
d is the distance = 1.0 cm = 1.0x10⁻² m
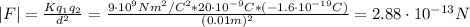
Now, we can find the acceleration:
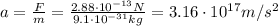
Therefore, the acceleration is 3.16x10¹⁷ m/s².
I hope it helps you!