Answer:
4.5 × 10⁻⁹
Step-by-step explanation:
Step 1: Write the reaction for the solution of CaCO₃
CaCO₃(s) ⇄ Ca²⁺(aq) + CO₃²⁻(aq)
Step 2: Convert the solubility of CaCO₃ from g/L to mol/L
We will use the following conversion factors:
- The molar mass of CaCO₃ is 100.09 g/mol.
- 1 L= 1000 mL.
- There are 0.00067 g of CaCO₃ per 100 mL of solution.
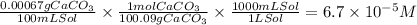
Step 3: Calculate the solubility product constant (Ksp)
To relate Ksp and the molar solubility (S), we need to make an ICE chart.
CaCO₃(s) ⇄ Ca²⁺(aq) + CO₃²⁻(aq)
I 0 0
C +S +S
E S S
The solubility product constant is:
Ksp = [Ca²⁺].[CO₃²⁻] = S² = (6.7 × 10⁻⁵)² = 4.5 × 10⁻⁹