Answer:
A) 0.831 M
B) 1.07 m
C) 26.2%
D) 0.0189
Step-by-step explanation:
A) First we convert the mass of Pb(NO₃)₂ to moles:
- 275 g ÷ 331 g/mol = 0.831 mol Pb(NO₃)₂
Then we divide it by the total volume (1.00 L) to calculate the molarity:
- 0.831 mol / 1.00 L = 0.831 M
B) We convert the grams of water to kilograms:
Then we divide the Pb(NO₃)₂ by the kilograms of water:
- 0.831 mol / 0.775 kg = 1.07 m
C) We divide the mass of Pb(NO₃)₂ by the total mass of the solution:
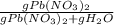 *100%
*100%
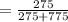 * 100% = 26.2%
* 100% = 26.2%
D) We calculate the moles of water:
- 775 g H₂O ÷ 18g/mol = 43.1 mol H₂O
Then we divide the Pb(NO₃)₂ moles by the total number of moles:
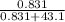 = 0.0189
= 0.0189