Answer:

Step-by-step explanation:
Hello!
In this case, since the definition of entropy in a random mixture is:
![\Delta S=-n_TR\Sigma[x_i*ln(x_i)]](https://img.qammunity.org/2021/formulas/chemistry/college/wgp4svlestwjjx9jh14grz16cvmfs26uiq.png)
For this silver-gold mixture we write:
![\Delta S=-(n_(Au)+n_(Ag))R\Sigma[(n_(Au))/(n_(Au)+n_(Ag)) *ln((n_(Au))/(n_(Au)+n_(Ag)) )+(n_(Ag))/(n_(Au)+n_(Ag)) *ln((n_(Ag))/(n_(Au)+n_(Ag)) )]](https://img.qammunity.org/2021/formulas/chemistry/college/k5tpsqv2wvxk8hj7vsqo1v63sgt5v6223a.png)
By knowing the moles of gold:
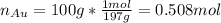
It is possible to write the aforementioned formula in terms of the variable
 representing the moles of silver:
representing the moles of silver:
![20(J)/(mol)=-(0.508+x)8.314(J)/(mol*K) \Sigma[(0.508)/(0.508+x) *ln((0.508)/(0.508+x) )+(x)/(0.508+x) *ln((x)/(0.508+x) )]](https://img.qammunity.org/2021/formulas/chemistry/college/6n98eiyci58bbq887kk77812l6mjqiaqwo.png)
Which can be solved via Newton-Raphson or a solver software, in this case, I will provide you the answer:
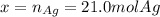
So the mass is:
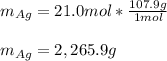
Best regards!