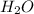Complete Question
Magnesium sulfate forms a hydrate with the formula
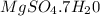 . What is the maximum amount of water (in grams) that can be removed from 15 ml of toluene by the addition of 200 mg of anhydrous magnesium sulfate? The molar mass of
. What is the maximum amount of water (in grams) that can be removed from 15 ml of toluene by the addition of 200 mg of anhydrous magnesium sulfate? The molar mass of
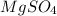 is 120.4 g/mol; H20 = 18 g/mol.
is 120.4 g/mol; H20 = 18 g/mol.
Answer:
The value is
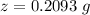 of
of
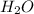
Step-by-step explanation:
From the question we are told that
The volume of toluene is
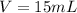
The mass of anhydrous magnesium sulfate is
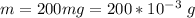
The formula of the hydrate is
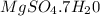
The molar mass of
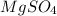 is
is
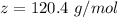
From the formula given we see that
1 mole of
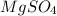 wil remove 7 moles of
wil remove 7 moles of
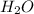 to for the given formula
to for the given formula
Hence
120.4 g (1 mole) will remove 7 moles (7 * 18 g = 126 g ) of
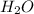 to for the given formula
to for the given formula
Therefore 1 g of
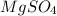 x g of
x g of
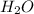
So
![x = (x]126 * 1)/( 120.4 )](https://img.qammunity.org/2021/formulas/chemistry/college/yem4umdprv0dqd966vzilkce0kevngu5ro.png)
=>
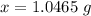
From our calculation we obtained that
1 g of
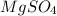 will remove
will remove
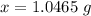 of
of
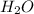
Then
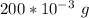 of
of
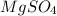 will remove z g of
will remove z g of
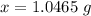 of
of
So
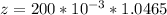
=>
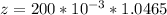
=>
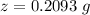 of
of