Answer:
d. kT ln 3
Step-by-step explanation:
Given;
k as Boltzmann constant
Let initial initial microstate, = Фi
Let the final microstate, = Фf = 3Фi
at constant temperature, T
The energy input to gas as heat is given by;
Q = TΔS = Tk(lnФf - lnФi)
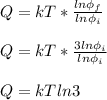
Therefore, the energy input to gas as heat is kT ln 3