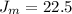Answer:
i
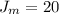
ii
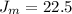
Step-by-step explanation:
From the question we are told that
The first temperatures is
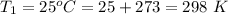
The second temperature is
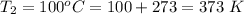
Generally the equation for the most highly populated rotational energy level is mathematically represented as
![J_(m) = [ (RT)/(2B)] ^{(1)/(2) } - (1)/(2)](https://img.qammunity.org/2021/formulas/chemistry/college/7a6u1hfdeyu7gvlwnmnmhz2q3v1dpj79d1.png)
Here R is the gas constant with value

Also
B is given as
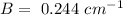
Generally the energy require per mole to move 1 cm is 12 J /mole
So
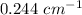 will require x J/mole
will require x J/mole
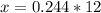
=>
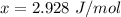
So at the first temperature
![J_(m) = [ (8.314 * 298 )/(2* 2.928 )] ^{(1)/(2) } - 0.5](https://img.qammunity.org/2021/formulas/chemistry/college/7ua5rfi89jds4s31lfsihxjt84qxlvtll0.png)
=>
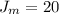
So at the second temperature
![J_(m) = [ (8.314 * 373 )/(2* 2.928 )] ^{(1)/(2) } - 0.5](https://img.qammunity.org/2021/formulas/chemistry/college/f29y8vtm6i1pnrg5gtu26q54fm2pnn2thh.png)
=>