Answer:
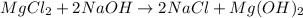
Step-by-step explanation:
Hello.
In this case, since aqueous magnesium chloride reacts with aqueous sodium hydroxide, a precipitate product, magnesium hydroxide is formed as well as aqueous sodium chloride as shown below:
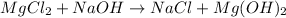
However, since there are two chlorine atoms at the reactants we need a 2 before NaCl on the products in order to balance it. Moreover, there one OH at the reactants and two OH at the products, that is why we also add a 2 before NaCl as shown below:
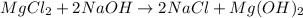
Best regards!