Answer:
Heat required to melt 42 g if ice is 13,860 J
Step-by-step explanation:
The amount of heat required to melt grams of water at 0°C is given as follows;
The mass of the ice (water), m = 42 g
The latent heat of fusion of ice Δ
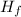 = 330 J/g
= 330 J/g
Therefore, heat required to melt 42 grams is given as follows;
Heat required to melt ice = Mass of the ice, m × The specific latent heat of fusion of the ice, Δ
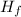
Heat required to melt 42 g if ice = m × Δ
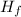 = 42 g × 330 J/g = 13,860 J
= 42 g × 330 J/g = 13,860 J
Heat required to melt 42 g if ice = 13,860 J.