Answer:
Test tube 1 0.10 M HCL
Test tube 2 0.10 M KOH
Test tube 3 0.10 M Na2CO3
Step-by-step explanation:
From the question we are told that
A few drops of the solutions from test tube 1 added to a similar volume of the solution in test tube 2 produces no visible reaction but the solution becomes warm
Generally this warmth is as a result of a reaction between an acid and a base and the acid is 0.10 M HCL and the base is 0.10 M KOH , the heat generated is know as the heat of neutralization,
The reaction is
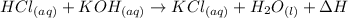
We are also told from the reaction that
A few drops of the solution from test tube 1 added to a similar volume of the solution in test tube 3 produces carbon dioxide gas.
Generally carbon dioxide gas is produced is as a result of a reaction between the acid HCl and Na2CO3.
The reaction is
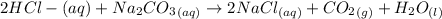
Hence from this explanation above we see that the solution in test tube 1 is 0.10 M HCL while solution in test tube 2 is 0.10 M KOH and then solution in test tube three is 0.10 M Na2CO3