Answer:
3 x 10²⁴ molecules
Step-by-step explanation:
A mole is just a name that we use to substitute a number, just like a dozen means 12, a mole means 6.022 x 10²³. This gives us the unit conversion of 6.022 x 10²³ molecules SO₂/1 mol SO₂.
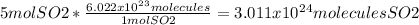
Round to the lowest number of significant figures to get 3 x 10²⁴ molecules SO₂