Answer:
The pH of the resulting hydrochloric acid solution is 1.896
Step-by-step explanation:
pH, short for Hydrogen Potential, is a measure of acidity or alkalinity that indicates the amount of hydrogen ions present in a solution or substance.
The pH scale goes from 0 to 14. Values less than 7 indicate the range of acidity and those greater than 7 indicate alkalinity or basicity. The value 7 is considered neutral.
pH is defined as the opposite of the base 10 logarithm or the negative logarithm of the activity of hydrogen ions or hydronium ions (H₃O)., whose equation is
pH = -log [H⁺] = -log[H₃O⁺]
Then you must know the concentration of [H⁺] or [H₃O⁺]
A strong acid is one that dissociates completely, therefore, a quantity of H + ions are transferred to the solution. Since HCl is a strong acid, then [HCl] = [H⁺]
Being molarity the number of moles of solute that are dissolved in a certain volume, it is defined as:
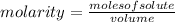
So you must know the number of moles of HCl. Being its molar mass equal to 36.45
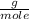 , then the following rule of three can be applied: if there are 35.45 grams of HCl in 1 mole, 0.9 grams of HCl in how many moles will they be?
, then the following rule of three can be applied: if there are 35.45 grams of HCl in 1 mole, 0.9 grams of HCl in how many moles will they be?
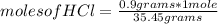
moles of HCl= 0.0254
Then, being the number of moles of solute 0.0254 and the volume 2 L, the molarity is:
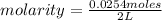
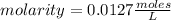
Then, the concentration of [HCl] is 0.0127
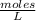 . So [HCl] = [H⁺] = 0.0127
. So [HCl] = [H⁺] = 0.0127
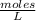 .
.
So the pH is calculated as:
pH = -log [H⁺]= -log 0.0127
pH= 1.896
The pH of the resulting hydrochloric acid solution is 1.896