Answer:
Mass of 0.5 moles CO2 is 22 and Mass of 5 moles of H2O is 90
Step-by-step explanation:
Moles =
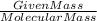
so value of moles is given 0.5 for co2 and 5 for h2o and the molecular mass of co2 is 44 and h2o is 18 so we put these values in
Mass = Moles x Molecular Mass
and we get the answer