Answer:
The concentration is
![[Cu^(2+)]_a = 10^(-10.269)](https://img.qammunity.org/2021/formulas/chemistry/college/x4ly2ztwrni7fnee4smvxpq0h5pt7hvfvr.png)
Step-by-step explanation:
From the question we are told that
The voltage of the cell is
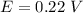
Generally the reaction at the cathode is
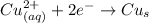 the half cell voltage is V_c = 0.337 V
the half cell voltage is V_c = 0.337 V
Generally the reaction at the anode is
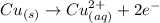 the half cell voltage is V_a = -0.337 V
the half cell voltage is V_a = -0.337 V
Gnerally the reaction of the cell is
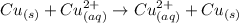
At initial the voltage is V = 0 V
Generally the voltage of the cell at 25°C is
![E = V - (0.0591)/(n) log ([Cu^(2+)] _a)/([Cu^(2+)]_c)](https://img.qammunity.org/2021/formulas/chemistry/college/mofjhzo1h7npxx6jt9fp8txd36z3y3ktpc.png)
Here n is number of of electron and it is 2
So from the question we are told that one cell has a concentration 1.5 x 10-3 M
Let assume it is
![[Cu^(2+)]_c](https://img.qammunity.org/2021/formulas/chemistry/college/e3z746pmct9h4wxxx0u77p8x1d16joaqs5.png)
So
![0.22= 0 - (0.0591)/(2) log ([Cu^(2+)] _a)/( 1.5 * 10^(-3) )](https://img.qammunity.org/2021/formulas/chemistry/college/fz1bmqp1s2c4xco13lviiyh0fybnjzu07t.png)
=>
![-7.445 = log ([Cu^(2+)] _a)/( 1.5 * 10^(-3) )](https://img.qammunity.org/2021/formulas/chemistry/college/5j1pafci6qjkvm9a6t47qvnzb5albt4bld.png)
=>
![-7.445 = log [Cu^(2+)_a] - log [1.5*10^(-3)]](https://img.qammunity.org/2021/formulas/chemistry/college/rnpnigkh97eq6tha0g7p3jrn4qm4hco54r.png)
=>
![-7.445 + log [1.5*10^(-3) = log [Cu^(2+)_a]](https://img.qammunity.org/2021/formulas/chemistry/college/plwch940ns4xnahpbnzlpw71qa445nc83l.png)
=>
![-7.445 - 2.824 = log [Cu^(2+)_a]](https://img.qammunity.org/2021/formulas/chemistry/college/2cmx3hn6uossjxqyp14nii7g6zzj48mejc.png)
Taking the antilog
=>
![[Cu^(2+)]_a = 10^(-10.269)](https://img.qammunity.org/2021/formulas/chemistry/college/x4ly2ztwrni7fnee4smvxpq0h5pt7hvfvr.png)
=>
![[Cu^(2+)]_a = 5.38 *10^(-11) \ M](https://img.qammunity.org/2021/formulas/chemistry/college/1druvo3axz6tgwpq0e4eq9mmaoca8131av.png)