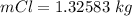Complete Question
Consider the rechargeable battery:
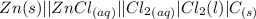
(a) Write reduction half-reactions for each electrode. From which electrode will electrons flow from the battery into a circuit if the electrode potentials are not too different from
 values
values
(b)
if the battery delivers a constant current of
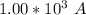 for 1.00 h , how many kg of
for 1.00 h , how many kg of
 will be consumed
will be consumed
Answer:
a
At the anode
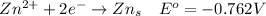
At the cathode
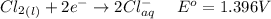
b
The value is
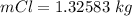
Step-by-step explanation:
Generally the half-reactions for each electrode is mathematically represented as
At the anode
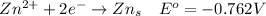
At the cathode
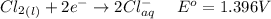
Generally from the question we are told that
The current is
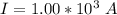
The time is
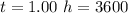
Generally the quantity of charge consumed is
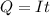
=>
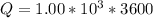
=>
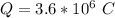
Generally the number of moles of electron consumed is
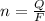
Here F is the faradays constant with value
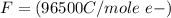
So
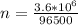
=>
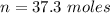
Generally the number of moles of
 consumed is mathematically represented as
consumed is mathematically represented as
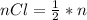
=>
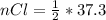
=>
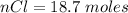
Generally the mass of
 consumed is mathematically represented as
consumed is mathematically represented as
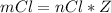
Here Z is the molar mass of
 is Z = 70.9 g/mole
is Z = 70.9 g/mole
So
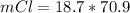
=>
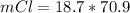
=>
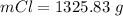
=>