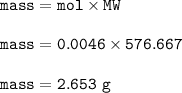Mass of Nd₂(SO₄)₃ : 2.653 g
Further explanation
Reaction
Nd₂Fe₁₄B + 17H₂SO₄ → Nd₂(SO₄)₃ + 14FeSO₄ + B + 17H₂
5 g Nd₂Fe₁₄B
MW = 2. 144,242 + 14. 55.845 + 10.811
MW = 288.484 + 781.83+10.811=1081.125 g/mol
mol :
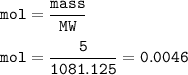
mol Nd₂Fe₁₄B = mol Nd₂(SO₄)₃=0.0046
so mass of Nd₂(SO₄)₃ :
MW Nd₂(SO₄)₃ = 2.144.242+3.32,065+12.15,999
MW = 288.484+96.195+191.988=576.667 g/mol