Answer:
3. NH3 is limiting
4. 90 g NO
Step-by-step explanation:
3. To find the limiting reactant, you have to convert from moles of reactant to moles of product. In this case, because we need to know how many grams of NO in the second question, we will use NO as our product. We use the ratios found in the chemical formula.
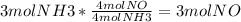
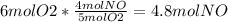
Because NH3 makes less moles of NO, it is the limiting reactant.
4. Now we need the molar mass to convert the moles of NO into grams of NO. The molar mass of NO is 30.01g/mol. This is the equation.
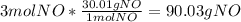
Because there are is only one significant figure in the original numbers, we round to one significant figure = 90 g NO.