Answer:
Find the limiting reactant.
Step-by-step explanation:
Using the ratio in the chemical formula, find the limiting reactant.
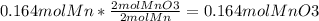
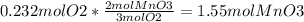
The second equation results in less mol of MnO₃, therefore it is the limiting reactant and changing the moles of O₂ to moles of MnO₃ which will give the theoretical yield which is 1.55 mol MnO₃.