Answer:
0.86 mol N
Step-by-step explanation:
To convert between grams and moles, you need the molar mass of the substance. The molar mass of nitrogen is 14.01g/mol. This is used as the unit converter.
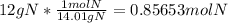
Then round the answer to the lowest number of significant figures = 0.86 mol N.