Answer:
133.33J/kg°C
Step-by-step explanation:
Given parameters:
Mass = 5kg
Temperature change = 3°C
Thermal energy = 2000J
Unknown:
Specific heat of the block = ?
Solution:
To find the specific heat capacity, use the formula below;
H = mCФ
where H is amount of heat
m is the mass
C is the specific heat capacity
Ф is the temperature change
Input the parameters and solve;
2000 = 5 x C x 3
C =
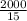
C = 133.33J/kg°C