Answer:
2.35 M
Step-by-step explanation:
Molarity is mol/L of solution. We have to convert the g to mol and the mL to L. G to mol uses the molar mass of the compound. The molar mass of NaNO₃ is 85.00g/mol.
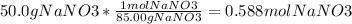
Then you have to convert mL to L.
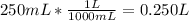
Now divide the mol by the L.
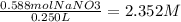
Round to the smallest number of significant figures = 2.35M