Step-by-step explanation:
Given that,
The frequency of electromagnetic spectrum is
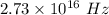
(A) Let the wavelength of this radiation is
 . We know that,
. We know that,
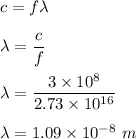
So, the wavelength of this radiation is
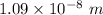 .
.
(B) Let E is the energy associated with this radiation. Energy of an electromagnetic radiation is given by :
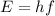
h is Planck's constant
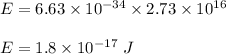
1 kcal = 4184 J
It means,
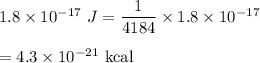
Hence, this is the required solution.