Answer:
V₂ = 450 cm³
Explanation:
The volume of a gas V held at a constant temperature in a closed container varies inversely with its pressure P.
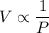
If V₁ = 600 cm³, P₁ = 300 mm hg, P₂ = 400 mm hg
We need to find a new volume. Using the above concept,
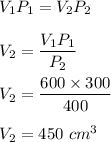
So, when the pressure is 400 mm hg, its volume is 450 cubic centimeters