Answer:
Dividing the silicon density by 1000 and then multiply it by 1000000.
Step-by-step explanation:
A kilogram equals 1000 grams and a cubic meter equals 1000000 cubic centimeters. Hence, we must divide the silicon density by 1000 and then multiply itby 1000000 to convert the value into kilograms per cubic centimeter. That is:
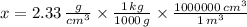
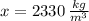
In a nutshell, we must multiply the density of silicon by 1000 to obtains its value in kilograms per cubic meter.