Answer: d) -705.55 kJ
Step-by-step explanation:
Heat of reaction is the change of enthalpy during a chemical reaction with all substances in their standard states.
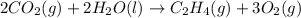
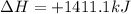
Reversing the reaction, changes the sign of

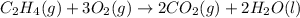
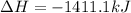
On multiplying the reaction by
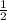 , enthalpy gets half:
, enthalpy gets half:
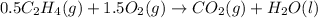
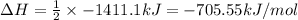
Thus the enthalpy change for the given reaction is -705.55kJ