The equation
4.94 x c . (Tm-23.6)=C.(23-6-21.9)+123.13 x c. (23.6-21.9)
Further explanation
The law of conservation of energy can be applied to heat changes, ie the heat received/absorbed is the same as the heat released
Q in = Q out
Heat can be calculated using the formula:
Q = mc∆T
A calorimeter is a device used to measure the specific heat of material
A metal is put into a calorimeter that contains water and there will be heat transfer:
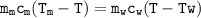
m = metal
w = water
T = the final temperature of the mixture
mass of metal =(Nickel) = 4.94 g
mass of calorimeter = 12.5 g
mass of water = 123.13 g (135.63 - 12.5)
The equation
Q released (metal) = Q absorbed(calorimeter+water)
- Qmetal = 4.94 x c . (Tm-23.60)
- Q calorimeter = C.(23-6-21.9) --> C = heat capacity of calorimeter
- Q water = 123.13 x c. (23.6-21.9)
The equation :
4.94 x c . (Tm-23.6)=C.(23-6-21.9)+123.13 x c. (23.6-21.9)