Answer:
The answer is 0.228 M
Step-by-step explanation:
To find the concentration of new solution we use the formula
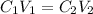
where
C1 is the concentration of the stock solution
V1 is the volume is the volume of the stock solution
C_2 is the concentration of the diluted solution
V2 is the volume of the diluted solution
Since we are finding the the concentration of new solution
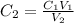
From the question
C1 = 0.575 M
V1 = 298 mL
V2 = 750 mL
We have
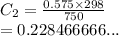
We have the final answer as
0.228 M
Hope this helps you