Answer:
The correct option is;
(B)
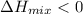 , solution shows negative deviation
, solution shows negative deviation
Step-by-step explanation:
The given parameters are;
The available volume of liquid A = 25 cm³
The available volume of liquid B = 20 cm³
The volume of the solution (mixture) = 44.8 cm³
Therefore, we have;
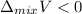
Which is one of the prerequisite for the formation of negative deviation
When a non-ideal solution shows negative deviation according to Raoult's Law, we have;
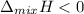 , we have more heat released due to new molecular interactions.
, we have more heat released due to new molecular interactions.