Answer:
1.586 J/g°C
Step-by-step explanation:
So, we have the formula
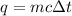 .
.
Since heat released by the metal is = to the heat absorbed by the water (because they eventually become the same temperature in solution), we can say
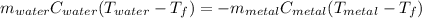
Plugging in, we get:
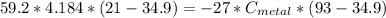
Solving, we get
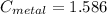 J/g°C.
J/g°C.