Solution :-
Given to us is the Reaction of Aluminium metal with Chlorine gas . That is ,
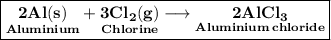
Now Mass of 1 mole aluminium = 27g . So mass of 2 moles of it would be 27×2 g = 54g .
Also , Mass of 1 mole of chlorine gas = 35.5 g . So mass of 2 Atoms will be 71g . And mass of 3 moles of atom will be 71 × 3g = 213g .
Now , use unitary method ;
213 g of Chlorine reacts with 54g of Aluminium.
1 g of Chlorine will react with
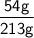 of Aluminium.
of Aluminium.
Hence 52.9 g of Chlorine will react with
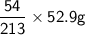 =
=
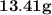 of Aluminium.
of Aluminium.