P = 11.133 atm (purple)
T = -236.733 °C(yellow)
n = 0.174 mol(red)
Further explanation
Some of the laws regarding gas, can apply to ideal gas (volume expansion does not occur when the gas is heated),:
- Boyle's law at constant T, P = 1 / V
- Charles's law, at constant P, V = T
- Avogadro's law, at constant P and T, V = n
So that the three laws can be combined into a single gas equation, the ideal gas equation
In general, the gas equation can be written
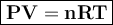
where
P = pressure, atm
V = volume, liter
n = number of moles
R = gas constant = 0.08206 L.atm / mol K
T = temperature, Kelvin
To choose the formula used, we refer to the data provided
Because the data provided are temperature, pressure, volume and moles, than we use the formula PV = nRT
T= 10 +273.15 = 373.15 K
V=5.5 L
n=2 mol

V=8.3 L
P=1.8 atm
n=5 mol

T = 12 + 273.15 = 285.15 K
V=3.4 L
P=1.2 atm
