Answer:
288 grams
Step-by-step explanation:
The amount of any radiative substance left after some time is calculated by
amount left =
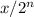
where x is the initial amount of substance
n is the number of half lives
one half live is the time in which amount of substance reduce to half of its initial value.
------------------------------------------------------
amount left = 4.5 grams
half life of iodine-125 = 60 days
total time in which substance reduced to 4.5 grams = 360 days
thus,
number of half life in 60 days = 360/60 = 6
thus, there are period of 6 half lives
in each half life the amount of substance reduces to its previous half
let the initial amount be x grams
using the formula
amount left =
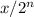
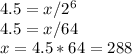
Thus, 288 grams was the initial amount of iodine-125