Answer:
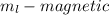
Step-by-step explanation:
The principal energy level, n, tells us which shell it is in and the total energy of the electron.
The angular momentum (azimuthal), l, tells us which sublevel/subshells the electron is in.
The magnetic, m sub l, tells us the orientation the electron is spinning.