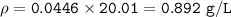NO can't be used to fill a balloon
Further explanation
Conditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure). At STP, Vm is 22.4 liters/mol.
Answer options that need to be added :
a. Ne
b. NO
c. NH₃
d. CH₄
e. HF
will float in air ⇒ element or compound to fill the balloon, its density must be less than < 1.285 g/L
We can use the ideal gas formula ta find density :
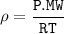
Because at STP, then the constant value is

So that the density is determined from the MW(molecular weight) of each element or compound
Ar = 20.1797 g/mol
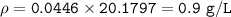
MW=30.006 g/mol
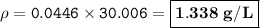
MW=17.0306 g/mol
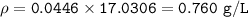
MW=16.04 g/mol
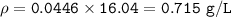
MW=20.01 g/mol