Answer:
Mass of copper oxide: 10g
Mass of copper: 8.882g
Mass of oxygen: 1.118g
Cu O
8.882 1.118
Divide by
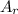 8.882÷63.5 1.118÷16
8.882÷63.5 1.118÷16
0.14 0.07
Divide by the lowest number 0.14/0.07 0.07/0.07
2 1
Check the ratio now, Cu has 2 atoms and O has 1 atom .
The formula is
