Answer:
Q = 67.04 [J]
Step-by-step explanation:
In order to solve this problem, we must use the following equation of thermal energy, which relates mass to specific heat and temperature change.
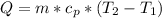
where:
Q = thermal energy [J]
m = mass = 0.5 [kg]
Cp = specific heat = 4,190 [J/kg°C]
T2 = final temperature = 65 [C]
T1 = initial temperature = 30 [C]
Now replacing:
Q = 0.5*4.190*(65 - 30)
Q = 67.04 [J]