Answer: The mass of
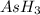 produced is, 1528.8 grams.
produced is, 1528.8 grams.
Explanation : Given,
Mass of
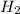 = 117.4 g
= 117.4 g
Molar mass of
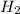 = 2 g/mol
= 2 g/mol
First we have to calculate the moles of
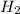 .
.
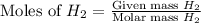
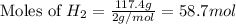
Now we have to calculate the moles of
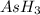
The balanced chemical equation is:
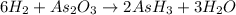
From the reaction, we conclude that
As, 6 moles of
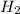 react to give 2 moles of
react to give 2 moles of
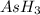
So, 58.7 moles of
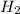 react to give
react to give
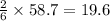 mole of
mole of
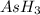
Now we have to calculate the mass of
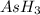
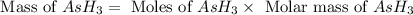
Molar mass of
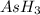 = 78 g/mole
= 78 g/mole
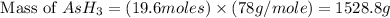
Therefore, the mass of
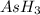 produced is, 1528.8 grams.
produced is, 1528.8 grams.