Answer:
40.03g/mole
Step-by-step explanation:
Given parameters:
Mass = 42.10 g/mole and abundance = 93.50%
Mass = 8.30 g/mole and abundance = 6.200%
Mass = 50.40 g/mole and abundace = 0.3000%
Find;
Average atomic mass = ?
Solution:
Average atomic mass = sum (mass x abundance)
Average atomic mass = (
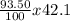 ) + (
) + (
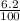 x 8.3) + (
x 8.3) + (
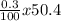 )
)
= 39.36g/mole + 0.52g/mole + 0.15g/mole
= 40.03g/mole