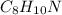Answer:
Step-by-step explanation:
We assume there is 100g of this first for simplicity. That means, there will be 80g of C, 8.33g of H, and 11.67g of N. Now, we need to find the moles of each:
C = 80/12.01 = 6.66
H = 8.33/1.008 = 8.26
N = 11.67/14.01 = 0.833
Then, we divide each one by the element with the lowest amount of moles which is N.
We get 6.66/0.833 = about 8 (this is for carbon)
We get 8.26/0.833 = about 10 (this is for hydrogen)
We get 0.833/0.833 = 1 (for Nitrogen)
Then we get the empirical formula