Answer :
Part 1: 4.93 moles of
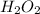 contains 9.86 moles of oxygen atoms.
contains 9.86 moles of oxygen atoms.
Part 2: 2.01 moles of
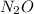 contains 2.01 moles of oxygen atoms.
contains 2.01 moles of oxygen atoms.
Explanation :
Part 1: 4.93 mol
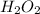
In 1 mole of
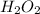 , there are 2 atoms of hydrogen and 2 atoms of oxygen.
, there are 2 atoms of hydrogen and 2 atoms of oxygen.
As, 1 mole of
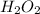 contains 2 moles of oxygen atoms.
contains 2 moles of oxygen atoms.
So, 4.93 moles of
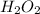 contains
contains
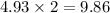 moles of oxygen atoms.
moles of oxygen atoms.
Thus, 4.93 moles of
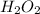 contains 9.86 moles of oxygen atoms.
contains 9.86 moles of oxygen atoms.
Part 2: 2.01 mol
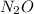
In 1 mole of
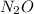 , there are 2 atoms of nitrogen and 1 atom of oxygen.
, there are 2 atoms of nitrogen and 1 atom of oxygen.
As, 1 mole of
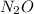 contains 1 mole of oxygen atoms.
contains 1 mole of oxygen atoms.
So, 2.01 moles of
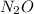 contains
contains
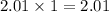 moles of oxygen atoms.
moles of oxygen atoms.
Thus, 2.01 moles of
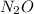 contains 2.01 moles of oxygen atoms.
contains 2.01 moles of oxygen atoms.