Answer:
The mass of chlorine to react completely with 5.0 moles of aluminum is 531.75 grams.
Step-by-step explanation:
Aluminum reacts with chlorine gas to form aluminum chloride through the following reaction, which is balanced (In a chemical equation, the number of atoms of each element in the reactants must be equal to the number of atoms of each element in the products In order to comply with the Law of Conservation of Mass, that is, in a chemical reaction the mass remains constant, that is, the mass that is consumed from the reactants is the same as that obtained from the products of the reaction):
2 Al + 3 Cl₂ ⇒ 2 AlCl₃
The rule of three is a way of solving problems of proportionality between three known values and one unknown, establishing a proportionality relationship between all of them. A proportionality is direct when one magnitude increases the other also does it, and if the magnitude decreases the other in the same way. In this case, the rule of three, knowing a, b and c and with x being the unknown, is applied as follows:
a ⇒ b
c ⇒ x
So
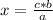
In this case the rule of three can be applied as follows: if by stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction) 2 moles of aluminum react with 3 moles of chlorine, 5 moles of aluminum with how many moles of chlorine do they react?
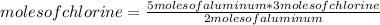
moles of chlorine= 7.5
Since the molar mass of the gas Cl2 is 70.9 grams/mole, you can apply the following rule of three: if 1 mole has 70.9 grams, 7.5 moles, how much mass does it have?
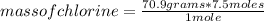
mass of chlorine= 531.75 grams
The mass of chlorine to react completely with 5.0 moles of aluminum is 531.75 grams.