Answer:
7.5 x 10²¹atoms
Step-by-step explanation:
Chemical formula of the compound:
C₁₃H₁₈O₂
Given mass = 200mg
Unknown:
Number of atoms in 200mg ibuprofen = ?
Solution:
We need to find the number of moles represented by this mass;
Number of moles =

Molar mass of C₁₃H₁₈O₂ = (12x13) + (1 x 18) + (16 x 2) = 206g/mol
Input the parameter and solve for the number of moles;
Number of moles =
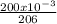 = 0.00097mol
= 0.00097mol
In 1 mole of C₁₃H₁₈O₂ there are 13 mole of carbon;
' So, in 0.00097mol, we will have 13 x 0.00097mol = 0.013mole
1 mole of an atom contains 6.02 x 10²³ atoms
0.013 mole of Carbon will contain 0.013 x 6.02 x 10²³ atoms
= 7.5 x 10²¹atoms