Given :
A volume of 80.0 mL of a 0.690 M
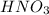 solution is titrated with 0.790 M KOH.
solution is titrated with 0.790 M KOH.
To Find :
The volume of KOH required to reach the equivalence point.
Solution :
We know, at equivalent point :
moles of
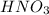 = moles of KOH
= moles of KOH
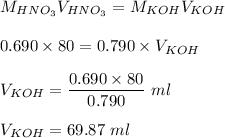
Therefore, volume of KOH required is 69.87 ml.
Hence, this is the required solution.