Answer:
1. The pH of 1.0 M trimethyl ammonium (pH = 1.01) is lower than the pH of 0.1 M phenol (5.00).
2. The difference in pH values is 4.95.
Step-by-step explanation:
1. The pH of a compound can be found using the following equation:
![pH = -log([H_(3)O^(+)])](https://img.qammunity.org/2021/formulas/chemistry/college/va1y2a3y2b1ctby5ywvb1ymwozqxskpaei.png)
First, we need to find [H₃O⁺] for trimethyl ammonium and for phenol.
Trimethyl ammonium:
We can calculate [H₃O⁺] using the Ka as follows:
(CH₃)₃NH⁺ + H₂O → (CH₃)₃N + H₃O⁺
1.0 - x x x
![Ka = ([(CH_(3))_(3)N][H_(3)O^(+)])/([(CH_(3))_(3)NH^(+)])](https://img.qammunity.org/2021/formulas/chemistry/college/jqjwo82drrannt41kix4dalkhplpqprmk7.png)
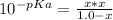
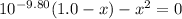
By solving the above equation for x we have:
x = 0.097 = [H₃O⁺]
![pH = -log([H_(3)O^(+)]) = -log(0.097) = 1.01](https://img.qammunity.org/2021/formulas/chemistry/college/snzleyb8r91gal5sokptcbsdhyd7yckxdp.png)
Phenol:
C₆H₅OH + H₂O → C₆H₅O⁻ + H₃O⁺
1.0 - x x x
![Ka = ([C_(6)H_(5)O^(-)][H_(3)O^(+)])/([C_(6)H_(5)OH])](https://img.qammunity.org/2021/formulas/chemistry/college/ct86oel8i7bm5y237lxpsutgyv9s5vc8b7.png)
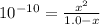
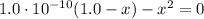
Solving the above equation for x we have:
x = 9.96x10⁻⁶ = [H₃O⁺]
![pH = -log([H_(3)O^(+)]) = -log(9.99 \cdot 10^(-6)) = 5.00](https://img.qammunity.org/2021/formulas/chemistry/college/pbnx0pf86390jfoocni7mvfa3geredp8rx.png)
Hence, the pH of 1.0 M trimethyl ammonium is lower than the pH of 0.1 M phenol.
2. The difference in pH values for the two acids is:
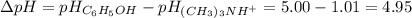
Therefore, the difference in pH values is 4.95.
I hope it helps you!