Answer:
The volume of the sample of gold is
16.51
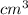
Step-by-step explanation:
The formula for density is:
D=
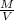 .
.
where:
D is density,
M is mass, and
V is volume.
Rearrange the density formula to isolate volume.
V=
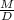
V=
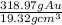
V= 318.97∅ ×
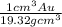 ← Multiply by the multiplicative inverse of the density.
← Multiply by the multiplicative inverse of the density.
V= 16.51 cm³ Au.