Answer:
0.088 M.
Step-by-step explanation:
Assuming that the temperature is 429 K, we can calculate the concentration of NO₂. Kc(429K) = 0.490.
The reaction is:
N₂O₄(g) ⇄ 2NO₂(g)
0.04 0.04
0.04 - x 0.04 + 2x
The constant of the reaction is:
![Kc = ([NO_(2)]^(2))/([N_(2)O_(4)]])](https://img.qammunity.org/2021/formulas/chemistry/college/uxmu3q0962u1ij6yi69pddmjd3p4sy8cct.png)
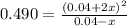
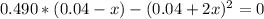
By solving the above equation for x we have:
x = 0.024 = [NO₂]
Hence the concentration of NO₂ is:
![[NO_(2)] = 0.04 + 2*0.024 = 0.088 M](https://img.qammunity.org/2021/formulas/chemistry/college/92ehz5ov23x50ib21r325s29uu44kkjbf6.png)
I hope it helps you!