Answer:
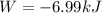
Step-by-step explanation:
Hello,
In this case, since the work at constant pressure is computed via:
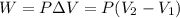
For an initial volume of 99.0 L and a final one of 98.0 L, accounting for a compression process at a pressure of 69.0 atm, we obtain a negative work, meaning that work is done on the gas, according to the first law of thermodynamics:
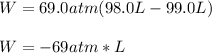
However, the work in kJ with three significant figures because the initial data have all three significant figures, is:
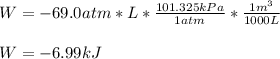
Best regards.