Answer:
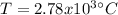
Step-by-step explanation:
Hello,
In this case, considering that the safe temperature may be computed via the ideal gas law as we now the pressure, mass and volume via the dimensions:
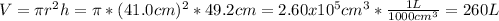
The pressure in atm is:
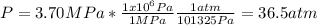
And the moles considering the mass and molar mass (66 g/mol) of dinitrogen difluoride (N₂F₂):
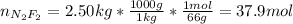
In sich a way, by applying the ideal gas equation, which is not the best assumption but could work as an approximation due to the high temperature, the temperature, with three significant figures, will be:
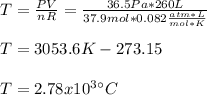
Best regards.