Answer:
(a). The decay constant is
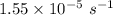
The half life is 11.3 hr.
(b). The value of N₀ is
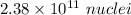
(c). The sample's activity is 1.87 mCi.
Step-by-step explanation:
Given that,
Activity
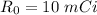
Time
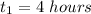
Activity R= 8 mCi
(a). We need to calculate the decay constant
Using formula of activity
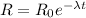
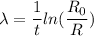
Put the value into the formula
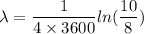
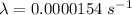
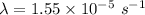
We need to calculate the half life
Using formula of half life
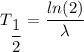
Put the value into the formula
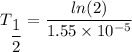
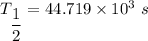
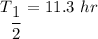
(b). We need to calculate the value of N₀
Using formula of
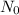
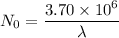
Put the value into the formula
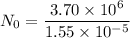
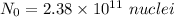
(c). We need to calculate the sample's activity
Using formula of activity
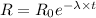
Put the value intyo the formula
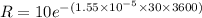
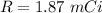
Hence, (a). The decay constant is
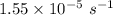
The half life is 11.3 hr.
(b). The value of N₀ is
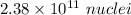
(c). The sample's activity is 1.87 mCi.